Physical Properties of Haloalkanes and Haloarenes
Physical Properties of Haloalkanes and Haloarenes: Overview
This topic covers concepts, such as Physical Properties of Haloalkanes and Haloarenes, Colour of Haloalkanes and Haloarenes, Melting and Boiling Points of Haloalkanes and Haloarenes, Density of Haloalkanes and Haloarenes, etc.
Important Questions on Physical Properties of Haloalkanes and Haloarenes
Order of hydrolysis of the following in increasing order is :
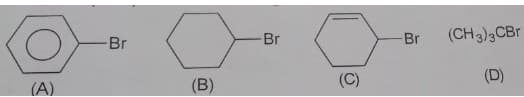
Which among the following will have highest density?
Which will have the highest boiling point
The increasing order of densities of the following alkyl halides is
i.
ii.
iii.
Which of the following order is incorrect?
Arrange the following compounds in order of their boiling point.
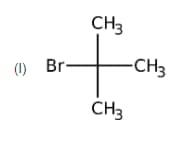
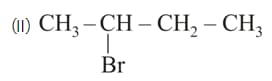
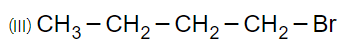
Among the following structures, in which of the following the magnitude of physical properties for compound I is lower than compound II.
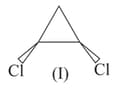 and
and 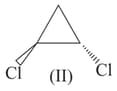
Which of the following will have maximum dipole moment and maximum boiling point respectively?
Order of boiling point in a group of isomeric alkyl halides is:
Among the following, hexachloroethane is also named as:
Which isomer of dichlorobenzene has the highest melting point?
Cotyledons are also called-
Which of the following compounds will have the highest melting point here?
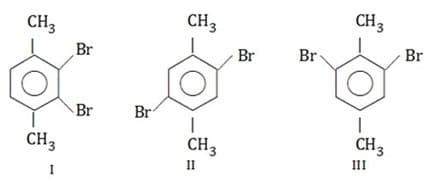
Arrange the following compounds in decreasing order of their boiling points
Alkyl halides are immiscible in water though they are polar because
Which of the following compounds will have highest melting point?
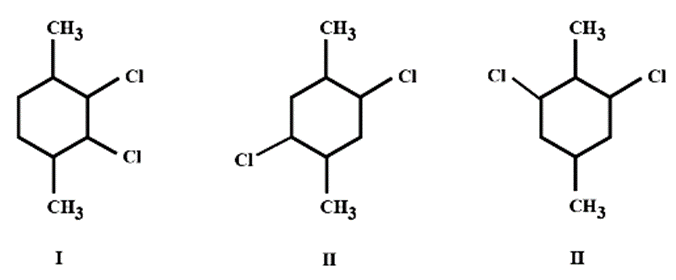
Arrange the following compounds in decreasing order of their boiling points
The dipole moments of and are in the order
The dipole moment of 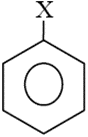 is . The dipole moment of
is . The dipole moment of 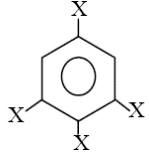 is
is
Which of the following is incorrect order?
